Next edition – Scientific programme
Following feedback from previous editions, courses of the 4th edition of the eSPARK summer school will be split into two categories: those which are mandatory and those which are elective. All students will be required to complete the mandatory courses, which will take place on the first day of the school. For the remainder of the school, students will take the elective courses that they chose upon admission into the school.
Note: since some elective courses may be more popular than others, those which do not receive sufficient interest may be cancelled. Therefore, the list of elective courses is a provisional guide to the scientific programme of this edition.
Mandatory courses
Experimental design in electrochemistry
This course covers the key aspects of experimental design that are encountered in most electrochemical experiments. We will check how potentiostat settings can affect voltammetry, discover how to apply iR compensation and prepare and test an Ag/AgCl reference electrode.

Demonstrators: Steven Linfield
Written by: Steven Linfield
Managed by: Steven Linfield
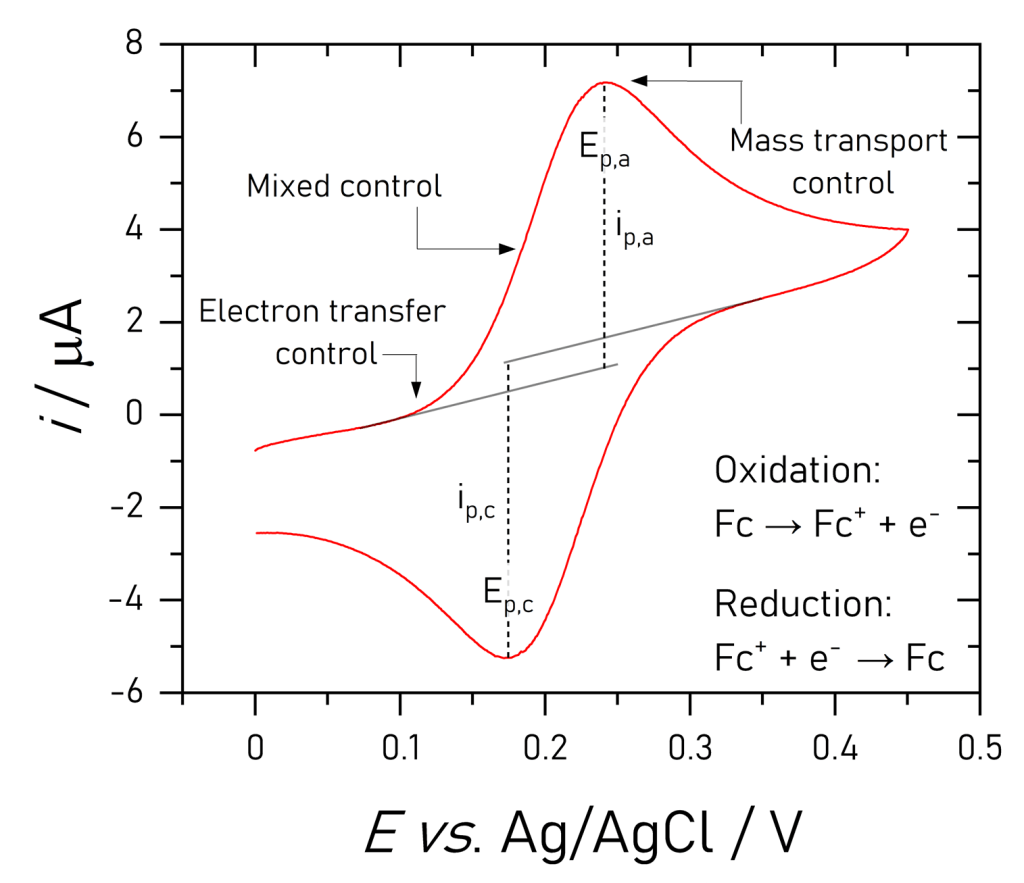
Cyclic voltammetry
In this exercise we will explore the shape of a typical cyclic voltammogram by far the most commonly used analytical method encountered in electrochemistry. Next we will compare the voltammetry of diffusing and adsorbed redox compounds
Demonstrators: Martin Jönsson-Niedziółka
Written by: Martin Jönsson-Niedziółka
Managed by: Martin Jönsson-Niedziółka
Elective courses
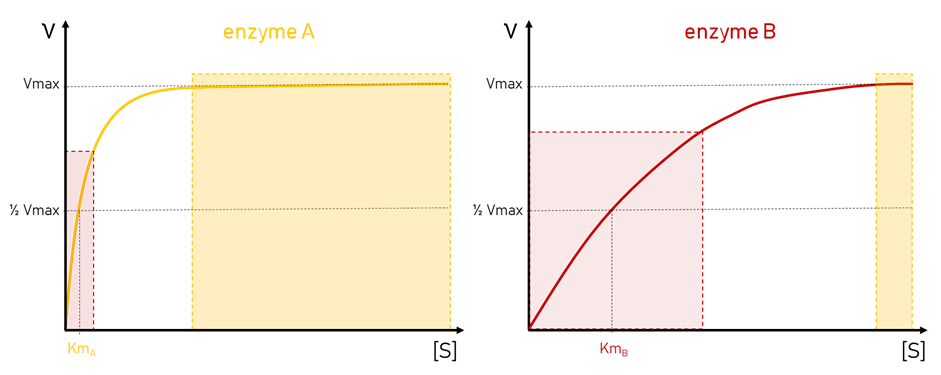
Biosensors
We will learn how to construct a simple glucose biosensor and characterize its function by constructing a calibration curve. Next we will analyze the impact of inhibitors and in the end measure glucose in real samples comparing our biosensor to a commercial glucometer.
Demonstrators: Karthika Kappalakandy Valapil
Managed by: Emilia Witkowska-Nery
Written by: Emilia Witkowska-Nery
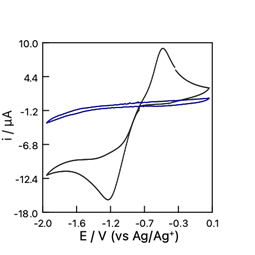
Non-aqueous electrochemistry
In this practical, we will study the electrochemistry in nonaqueous solutions. We will learn how to use an internal reference compound with a pseudo- reference electrode. Later during cyclic voltammetry experiments we will discover how water contamination and the presence of oxygen can affect the potential window and influence the number of reduction steps observed for a molecule like fullerene.
Demonstrators: Steven Linfield
Managed by: Steven Linfield
Written by: Bren Mark Felisilda
Simulation of electrode processes

Demonstrators: Martin Jönsson-Niedziółka, Steven Linfield
Managed by: Martin Jönsson-Niedziółka, Steven Linfield
Written by: Martin Jönsson-Niedziółka, Steven Linfield
Microelectrodes
The aim of this practical is to learn how to prepare a glass sealed voltammetric microelectrode using easily accessible materials and equipment. We will visually assess the size and quality of the electrode using optical microscopy, renew its surface through manual and electrochemical polishing. In the end we will learn how to estimate its size using steady-state voltammetry.
Demonstrators: Ariba Aziz
Managed by: Wojciech Nogala
Written by: Wojciech Nogala
Scanning electrochemical microscopy
This practical aims to familiarize you with the basics of scanning electrochemical microscopy. We will measure the size of the active part of the SECM tip (probe) and the size of its insulating sheath using positive and negative feedback approach curves. In the end we will assess the local heterogeneous rate constant by fitting a feedback mode approach curve.
Demonstrators: Ariba Aziz
Managed by: Wojciech Nogala
Written by: Wojciech Nogala
Potentiometry
During this experiment, we will prepare two types of ion-selective electrodes (with internal electrolyte and solid state) and verify their performance. We will assess their sensitivity and linear range and in the end determine selectivity coefficients for different ions.

Demonstrators: Elżbieta Jarosińska
Managed by: Emilia Witkowska-Nery
Written by: Emilia Witkowska-Nery
Ion transfer voltammetry
This practical aims to familiarize you with the basics of classical polarizable interface ion-transfer setup. We will learn how to assemble the ITIES cell and distinguish between ion-transfer and electron transfer events.

Demonstrators: Emilia Witkowska-Nery
Managed by: Emilia Witkowska-Nery
Written by: Emilia Witkowska-Nery
Three-phase junction electrochemistry
In this exercise we will learn the basics of three-phase junction electrochemistry performing experiments in a droplet system and with a wire setup. We will check what is how the type of ion and its concentration influence the observed ion-transfer process.

Demonstrators: Emilia Witkowska-Nery
Managed by: Emilia Witkowska-Nery
Written by: Emilia Witkowska-Nery
Molecularly imprinted polymers (MIPs)
In this exercise we will polymerize three different polymers and check their impact on the electrochemical signal. We will characterize the layers using SEM and AFM. In the end prepare a calibration curve with a molecularly imprinted polymer biosensor (provided by the organizers).

Demonstrators: Maciej Cieplak
Managed by: Maciej Cieplak
Written by: Piyush Sindhu Sharma
Microfluidic devices
During this exercise we will prepare indium-tin oxide (ITO) band electrodes and assemble a microfluidic system with a PDMS channel. Next we will observe the influence of flow rate on the measured current in cyclic voltammetry.

Demonstrators: Marcin Szymon Filipiak
Managed by: Marcin Szymon Filipiak
Written by: Marcin Szymon Filipaik
Low-cost electrochemistry systems
During this exercise we will prepare simple low-cost electrochemical setups made from paper and double sided tape. We will check how they can be used for prototyping and as Point-of-care systems by testing their analytical parameters.

Demonstrators: Emilia Witkowska-Nery
Managed by: Emilia Witkowska-Nery
Written by: Emilia Witkowska-Nery
Rotating disc electrodes
In this practical we will learn about most traditional electrochemical hydrodynamic setup the rotating disc electrode. We will observe a generation-collection process using a rotating ring disc electrode (RRDE) and calculate the limiting and kinetic current using Koutecky-Levich equation.

Demonstrators: Marcin Szymon Filipiak
Managed by: Marcin Szymon Filipiak
Written by: Marcin Szymon Filipiak
Pulsed voltammetry techniques
We will explore differential pulse and square-wave voltammetry and they use in analytical electrochemistry. Such techniques can be used to minimize background charging currents, therefore first we will learn how to prepare screen-printed electrodes, that are quite porous and can better demonstrate advantages of those techniques.

Demonstrators: Martin Jönsson-Niedziółka
Managed by: Martin Jönsson-Niedziółka
Written by: Martin Jönsson-Niedziółka
Electrochemical impedance spectroscopy (EIS)
During this exercise you will understand impedance of simple elements of electric circuits and record spectra of basic equivalent circuits of electrochemical cells. Next you will determine the electrode process rate constant and electrical double layer capacitance in a simple electrochemical system.

Demonstrators: Ariba Aziz
Managed by: Wojciech Nogala
Written by: Wojciech Nogala
Solar cells
In this practical we will fabricate a perovskite photovoltaic device. After cleaning of the ITO/FTO substrate we will deposit the electron transport layer, the perovskite layer and the hole transport layer. After deposition of the metal contacts we will test and characterize the device.

Demonstrators: Jatin Yadav
Managed by: Daniel Prochowicz
Written by: Muhammed Ans
Spectroelectrochemistry
Demonstrators: Wojciech Nogala and Gonzalo Angulo
Managed by: Gonzalo Angulo
Written by: Gonzalo Angulo